Introduction:Transplantation-associated thrombotic microangiography (TA-TMA) is a disorder that causes severe complications post-hematopoietic cell transplantation (HCT). Diagnosing TA-TMA is challenging due to non-standardized criteria. The American Society for Transplantation and Cellular Therapy (ASTCT) has proposed new consensus diagnostic criteria for standard risk (ASTCT-SR) and high risk (ASTCT-HR) but their correlation with older criteria based on microangiopathic hemolytic anemia (MAHA) or diagnostic complement biomarkers used in previous TA-TMA studies, such as levels of the terminal complement activation product sC5b-9 and the alternative complement pathway activation product Factor Ba, is unknown. Using data and samples from an ongoing prospective pediatric cohort study, we compared the two definitions and their associations with biomarkers.
Methods:Patients aged 0 to 18 years who underwent first allogeneic HCT 2021-2023 at Texas Children's Hospital were included in the clinical and biomarker study. Those who did not have at least 100-day post-HCT event-free follow-up were excluded. Two different definitions for TA-TMA were applied. Clinical TMA (cTMA) was diagnosed if a patient 1) met 4/4 concurrent criteria of MAHA (schistocytosis, thrombocytopenia, anemia, and elevated lactate dehydrogenase (LDH)) at least twice in 14 days and 2) clinically adjudicated to exclude known mimics or alternative causes of TMA. In contrast, ASTCT-HR TMA was diagnosed if a patient 1) met 4/7 non-concurrent criteria (schistocytosis, thrombocytopenia, anemia, elevated LDH, elevated blood pressure, elevated urinary protein excretion, and elevated sC5b9) at least twice in 14 days and 2) had evidence of LDH >2x normal, proteinuria ≥1 mg/mg, elevated sC5b9, acute graft-versus-host disease, active bacterial/viral infection, or end-organ damage. Cumulative incidence of TA-TMA was assessed by the competing risk method. Overall survival was compared by the Kaplan Meier estimator and log-rank test. Biomarker levels for samples collected at day 15 (+/- 14 days) were compared using the t-test.
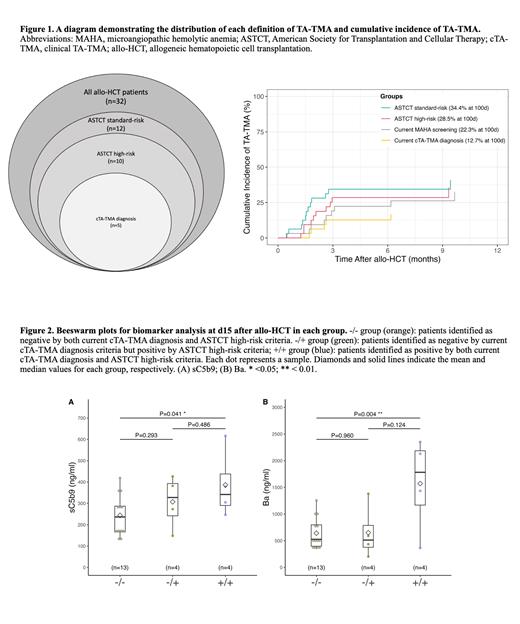
Results:A total of 32 patients met the inclusion criteria. The median age was 5.7 years (range 1.1-18.8) and most were White (68.8%) and Hispanic (56.2%). Malignant disease was the indication for HCT in 50% of patients. While the classic MAHA-based cTMA definition had an incidence of 12.7%, the newly proposed ASTCT-HR definition doubled it to 28.5% by day-100 (Figure 1). In contrast to patients with concordant TA-TMA diagnosis (+/+) who had significantly worse post-transplant survival (p=0.0047) compared with those with concordant non-TMA (-/-), those reclassified as TA-TMA by the new definition only (-/+) had an unexpectedly good prognosis (100% survival at day 100) despite the lack of TMA-directed therapy.
Longitudinal biomarker analysis was conducted on 29 patients. The highest average plasma levels of sC5b-9 and Ba during the first 100 days were observed in patients meeting both cTMA and ASTCT-HR criteria (+/+). When focused on early biomarkers measured around day 15, sC5b-9 (P=0.041) and Ba (P=0.004) were significantly elevated in the doubly positive TMA group (+/+) vs. non-TMA group (-/-) (Figure 2). We did not observe significant differences for ASTCT-HR singly positive (-/+) vs. non-TMA group (-/-).
Conclusions:In the current prospective clinical and biomarker study, the recently proposed ASTCT-HR definition was more sensitive but less prognostic than the traditional cTMA definition for TA-TMA. Patients who solely met the ASTCT-HR diagnostic criteria but not the cTMA criteria had benign complement biomarker profiles and excellent survival prognosis without TMA-directed therapy. While clinicians need to be vigilant for this infrequent yet potentially severe complications, it is crucial that diagnostic criteria accurately identify affected patients given the expense and potential complications of treatment. We recommend ongoing validations of the new consensus diagnostic criteria from other prospective cohort studies with larger sample sizes and extended follow-up.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal